 Cendres+Métaux SA won the prestigious Swiss Technology Award 2014 in the category Sustainability Leader. The Swiss Technology Award honours outstanding innovations and developments, which exhibit above-average market potential and great opportunities for growth. Prizes were awarded to a total of three companies competing in three different categories: Inventors, Start-Ups and Sustainability Leaders.
Cendres+Métaux SA won the prestigious Swiss Technology Award 2014 in the category Sustainability Leader. The Swiss Technology Award honours outstanding innovations and developments, which exhibit above-average market potential and great opportunities for growth. Prizes were awarded to a total of three companies competing in three different categories: Inventors, Start-Ups and Sustainability Leaders.
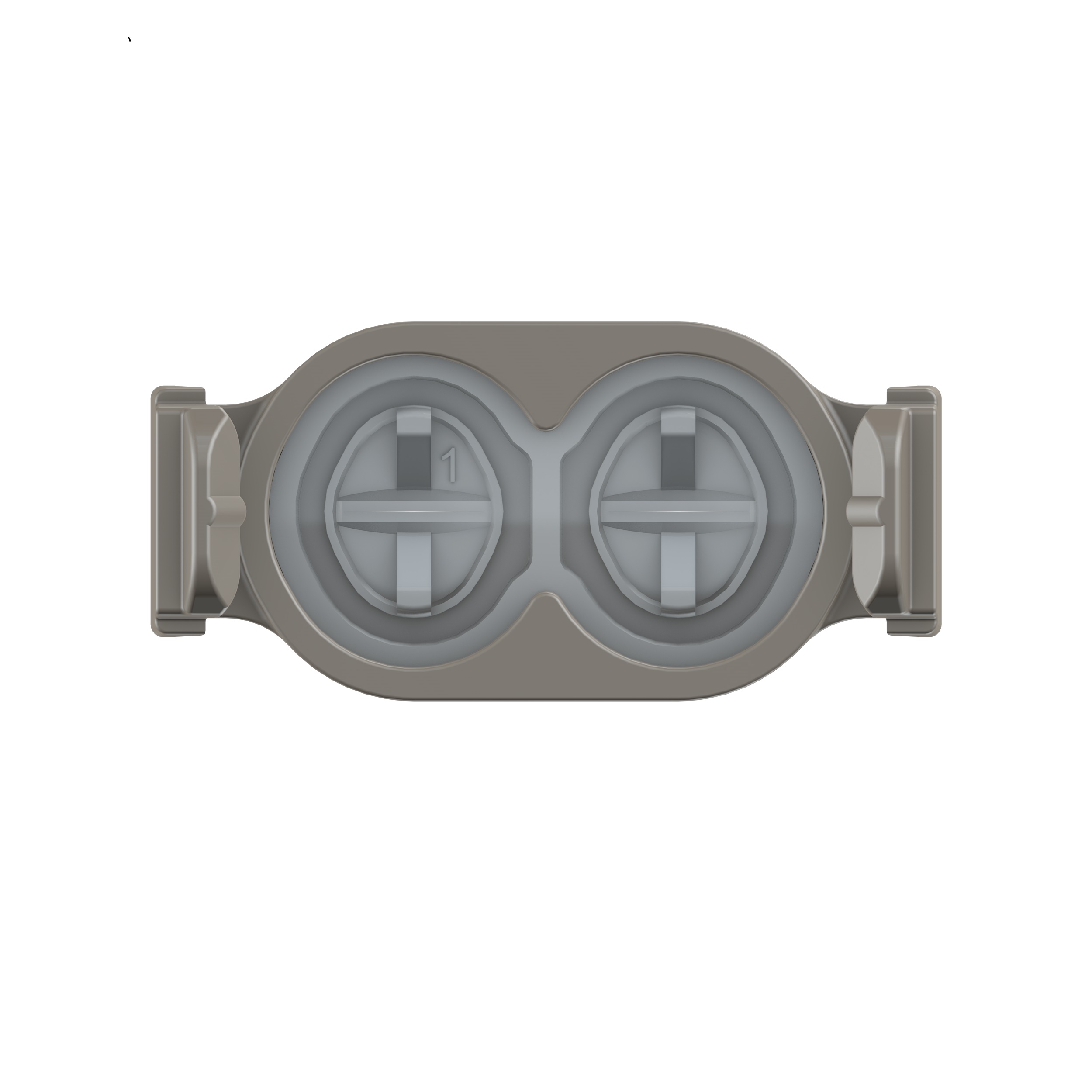
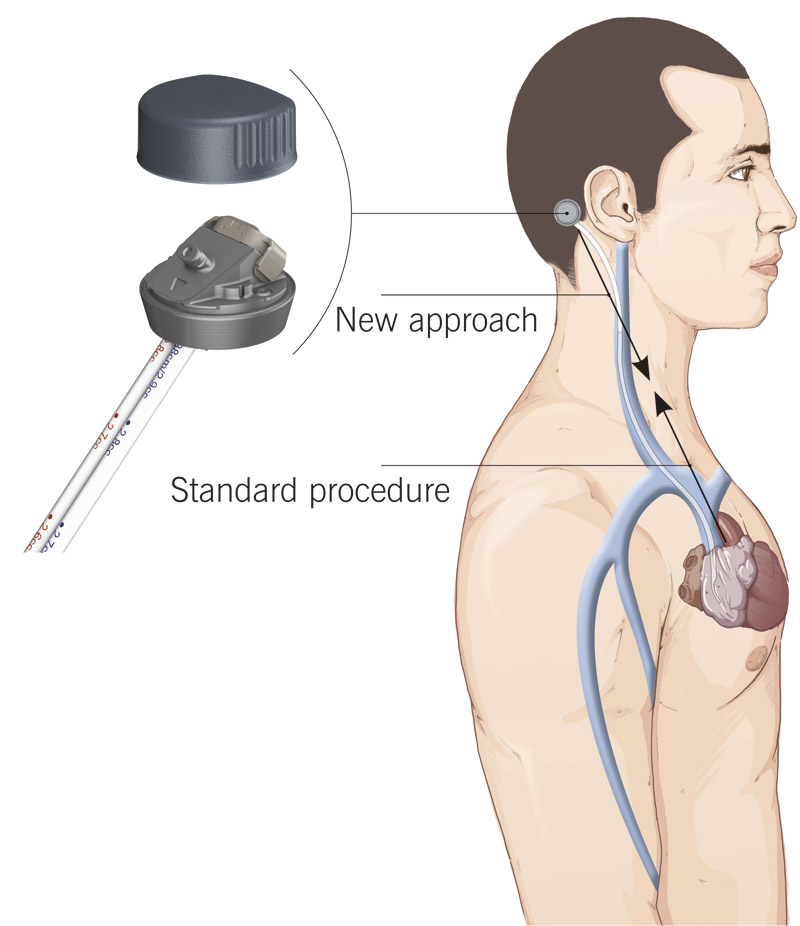
Cendres+Métaux developed a novel vascular access – the so called “Bone Anchored Port”. It is the first percutaneous, bone anchored and therefore permanent vascular access for chronic haemodialysis therapy in patients with renal failure.
The catheter is tunneled under the skin, enters the internal jugular vein and ends in the right atrium of the heart. The novelty of the access lies in its location (retro auricular on petrous bone) and its bone fixation. Compared to most of today’s access methods, the Bone Anchored Port provides more convenience to the patient: no needle stinging, both hands are available during dialysis, and most importantly: better protection from infections due to its location on the head where infections are typically very rare. Also the risk of thrombosis is reduced due to a straighter conduit of the catheter for improved haemodynamics.
The class III medical device reached Swiss FDA approval to start clinical trials by the end of last year. The first patients are now being recruited at the University hospital in Berne.
Read more about the device: BAP_System
MER-Europe and their partner Specialty Silicone Fabricators have been involved with this project from the beginning, developing the silicone access valve & seal as well as the internal catheter conduit which were custom-tailored to optimally serve their purpose of operating the device as safely and comfortably as possible. The reason that for this specific part Cendres+Métaux chose to work with Silicone is that Silicone rubber has been used in medical implants for more than 50 years. It has a proven track record as an inert and stable biomaterial.
SSF is a manufacturer of custom precision silicone components and OEM products for the medical device industry. The most modern and sophisticated techniques are utilized to mold, extrude and calender products to your exacting tolerances. A major part of their business is for long term implant applications and therefore SSF has built up a lot of expertise in these types of critical components and products. SSF is well known for their quality, broad capabilities and great customer service.
Update: MER does not represent this company anymore. However, if you are interested in the mentioned capabilities – please do not hesitate to contact us and we will be pleased to refer you.
Images: © Cendres+Métaux SA